Salt Crystal Shamrock Science Experiment: A Magical St. Patrick’s Day Activity for Kids

A salt crystal shamrock science experiment offers a hands-on way to combine seasonal creativity with simple chemistry. This St. Patrick’s Day science experiment for kids uses common materials to grow sparkling salt crystals on a shamrock-shaped pipe cleaner. The process highlights concepts like crystal formation, evaporation, and saturation in a way that feels magical while remaining grounded in real science.
This post contains affiliate links. I earn a small commission if you choose to purchase, at no extra cost to you!

This St. Patrick’s Day activity for kids works equally well in homeschool lessons or in classroom science centers. The slow transformation of the shamrock maintains interest over hours or even days while encouraging patience and observation. With minimal setup and a clear scientific outcome, this St. Patrick’s Day STEM activity fits neatly into early science learning plans.
St. Patrick’s Day Science Experiment for Kids Using Salt Crystals

Salt crystal experiments rely on a supersaturated solution. When warm water holds more dissolved salt than it can keep at room temperature, the excess salt forms visible crystals as the water cools and evaporates. Pipe cleaners act as an ideal structure for crystal growth because the fuzzy fibers give salt particles many places to attach.
Shaping the pipe cleaners into shamrocks adds a seasonal twist while reinforcing fine motor skills and creative thinking. The long stem allows the shamrock to hang freely in the solution without touching the sides of the jar, which supports even crystal growth.
Supplies for Salt Crystal Shamrock Science Experiment

- Green pipe cleaners
- Pencil or wooden skewer
- Clear glass jar
- Water
- Table salt
- Saucepan
- Stove or hot plate
- Spoon for stirring
Optional additions include magnifying glasses for observation or a notebook for recording changes over time.
Books To Read After Your St. Patrick’s Day Experiment
How to Make a Salt Crystal Shamrock

Step 1: Shape the Shamrock
Bend green pipe cleaners into a simple shamrock shape with three rounded loops forming the leaves. Twist the ends together to secure the shape. Add a long stem extending downward from the shamrock. The stem should be long enough to suspend the shamrock in the jar without touching the bottom or sides.
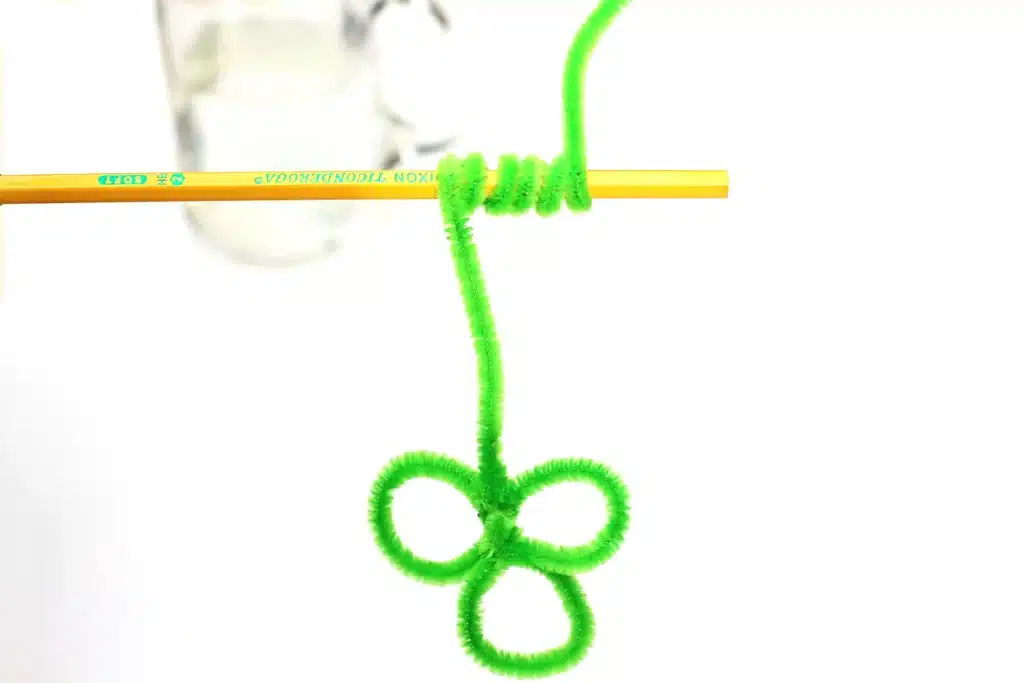
Step 2: Attach to the Pencil
Wrap the top of the pipe cleaner stem around a pencil. Adjust the length so the shamrock hangs freely when the pencil rests across the opening of the jar. Set this piece aside while preparing the solution.

Step 3: Prepare the Salt Crystal Water
Pour water into a saucepan and add salt. Heat the water until it begins to boil. Continue adding salt until it dissolves fully. Keep adding salt until a thin layer of crystallized salt forms on the surface of the boiling water. The top may appear icy or cloudy, which signals that the solution has reached saturation. Kids shouldn’t help with this step, due to the high heat of the water.

Step 4: Transfer the Solution
Carefully pour the hot saltwater solution into the clear jar. Adult supervision is required during this step due to the high temperature of the liquid.
Step 5: Suspend the Shamrock
Place the pencil across the top of the jar so the shamrock hangs in the saltwater. The shamrock should remain fully submerged without touching the sides or bottom of the container.

Step 6: Observe Crystal Growth
Leave the jar undisturbed for several hours. Small crystals begin forming along the pipe cleaner fibers as the solution cools. For larger crystals, allow the jar to sit overnight or longer.
The Science Behind Salt Crystal Growth

This St. Patrick’s Day science experiment for kids demonstrates supersaturation, evaporation, and crystallization. Warm water holds more dissolved salt than cool water. During heating, salt dissolves until the solution reaches a saturation point. Once cooling begins, the excess salt no longer stays dissolved.
As water slowly evaporates, salt particles reconnect and arrange into solid crystal structures. The pipe cleaner provides a framework where salt molecules gather and grow. Each fuzzy fiber acts as a potential starting point, allowing crystals to form evenly across the shamrock shape.
This process models real-world crystal formation, similar to how minerals form in nature. Observing this formation encourages scientific thinking through prediction, observation, and explanation.
Educational Benefits of This St. Patrick’s Day Activity for Kids
- Supports early chemistry concepts such as solutions and saturation
- Encourages patience and long-term observation
- Builds fine motor skills during shamrock construction
- Reinforces seasonal learning through themed science
- Promotes curiosity and hands-on exploration
- Strengthens understanding of cause-and-effect relationships
This St. Patrick’s Day STEM activity blends creativity with scientific reasoning, offering meaningful learning through an approachable shamrock science activity.
Tips for Best Crystal Results

- Use very hot water to dissolve the maximum amount of salt.
- Avoid disturbing the jar once the shamrock is placed inside.
- Ensure the shamrock does not touch the container sides.
- Allow extra time for thicker crystal layers to develop.
Crystal growth varies based on temperature, humidity, and salt concentration, which creates opportunities for discussion and comparison.
St. Patrick’s Day Science Experiment for Kids at Home or School
A salt crystal shamrock science experiment fits easily into seasonal lesson plans. This St. Patrick’s day activity for kids adds a visual element that keeps attention focused while reinforcing scientific concepts. Displaying the finished shamrock allows learners to revisit observations and reflect on the growth process.
Pairing this shamrock science activity with journaling, drawing, or magnified viewing deepens engagement. The combination of art and science creates a memorable experience rooted in exploration and discovery. Growing crystals may seem like almost magic, but the only magic here is the growing love for science in the children’s hearts.
More St. Patrick’s Day Fun!
- Gold Coin Catapults: A St. Patrick’s Day STEM ActivityGold coin catapults are a fun St. Patrick’s Day STEM activity for kids. Build, launch, and learn physics with popsicle sticks and coins.
- Rainbow Toilet Paper Roll Streamer: A St. Patrick’s Day Craft for KidsCreate rainbow toilet paper roll streamers. It’s a fun St. Patrick’s Day craft for kids that’s colorful, easy, and perfect for classroom or at-home fun.
- Salt Crystal Shamrock Science Experiment: A Magical St. Patrick’s Day Activity for KidsTry this salt crystal shamrock science Experiment for a simple St. Patrick’s Day activity that combines science with a fun shamrock crystal-growing project.
- Paper Leprechaun Trap: A DIY Leprechaun Trap for St. Patrick’s Day FunThis paper leprechaun trap is a fun and simple St. Patrick’s Day craft for kids that uses basic supplies to encourage creativity and imaginative pretend play.
- Spread Smiles All Year Long with These Adorable Seasonal Lunch Box NotesBrighten your child’s day with these seasonal holiday lunchbox notes—a fun and easy way to add joy, encouragement, and festive cheer to every meal! This collection of printable holiday lunchbox notes includes designs for Christmas, Valentine’s Day, Halloween, Easter, and more, making it perfect for school lunches, snacks, or surprise notes at home.
- St. Patrick’s Day Gratitude Writing Craft FREEBIEPractice gratitude this March with this FREE St. Patrick’s Day Gratitude Writing Craft! Your kids will love this fun writing craft to celebrate St. Patrick’s Day.
- The Importance of Name Crafts in Early Childhood Learning + A March FreebieLearn all about why Name Crafts are so important in early elementary school and get a FREE March name craft!












